|
Our lab is addressing these broad questions by
studying the development of zebrafish skeletal muscle. We
are working on muscle development because muscle is a
very abundant and easily accessible tissue, and also
because diseases of muscle development are debilitating
and common childhood diseases. We work on zebrafish
because they are readily accessible for experimental
manipulations throughout development and because a
genetic approach to studying development is feasible in
this vertebrate.
Vertebrate muscle precursors derive from a transient embryonic tissue known as the dermomyotome. The dermomyotome contains proliferating cells We have recently demonstrated that embryos of species in all known vertebrate classes contain a dermomyotome (Devoto, et al., 2006; Stellabotte and Devoto, 2007). We have extensively characterized the origin and the fate of zebrafish dermomyotome (Stellabotte, et al., 2007), and examined the development of the dermomyotome into muscle fibers.
Muscle fibers in most vertebrates can be broadly classified as either slow or fast. Slow muscle fibers contract slowly and they fatigue slowly; in fish, slow muscle is used for steady cruising through the water. Fast muscle fibers contract fast and fatigue fast; they are used to power rapid escape responses, such as when a predator appears.
|
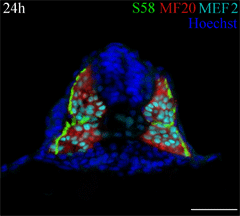 This cross section of a 24 hour old zebrafish embryo was stained with three antibodies to identify muscle fiber types. A monolayer of embryonic slow muscle fibers (yellowish green) is on the surface of the myotome, surrounding the deeper fast muscle fibers (red). All muscle nuclei are teal. Other nuclei in the embryo are labeled blue. This cross section of a 24 hour old zebrafish embryo was stained with three antibodies to identify muscle fiber types. A monolayer of embryonic slow muscle fibers (yellowish green) is on the surface of the myotome, surrounding the deeper fast muscle fibers (red). All muscle nuclei are teal. Other nuclei in the embryo are labeled blue.
|
|
Our long-term goal is to understand how cell-cell interactions
regulate the relative positions and proliferation of distinct
skeletal muscle cell types during development. Within the trunk,
two cell types, adaxial and lateral presomitic, can be
unambiguously identified in the presomitic mesoderm. Adaxial cells
are large, cuboidal cells adjacent to the notochord which will
develop into slow muscle fibers, while lateral presomitic cells
are smaller and more irregular cells in the segmental plate which
will give rise to both fast muscle and sclerotome (Devoto
et al., 1996). One or more members of the hedgehog (Hh) gene
family triggers specification of two different types of slow
muscle fibers: muscle pioneers and non-muscle pioneers (Du,
et al., 1997). Finally, ectopic expression of a member of the
BMP4 gene family in the notochord inhibits development of the
muscle pioneer class of slow muscle fibers, without affecting
development of either the non-muscle pioneer slow muscle fibers or
the fast muscle fibers (Du, et al.,
1997).
The role of Hh in embryonic slow muscle development
Zebrafish slow muscle development offers a very powerful
system in which to identify new genes involved in Hedgehog
signaling, and to test the function of known genes. We have tested
whether Hh is required for slow muscle development in two ways.
First, we have shown that pharmacological elimination of Hh
signaling blocks the development of embryonic slow muscle fibers.
Second, we have begun characterizing a new gene that we have named
slow-muscle-omitted (smo). smo mutant embryos have a nearly
complete loss of slow muscle fibers. smo function is
required in muscle precursors for them to respond to signaling
from the notochord, and thus this gene is a good candidate for
genes involved in notochord signaling. We have proposed that
smo is the zebrafish homologue of the Hh receptor complex
protein Smoothened, and that smu is required for all Hh
signal transduction. See Barresi, et al., 2000, for more information.
We have also shown that all other genes which are required for
Hh or midline signaling are required for slow muscle development
(Stickney, et al., 2000).
Muscle growth, larval development, and fiber type
identity
After these initial 20 muscle fibers per somite have developed, there is a tremendous growth of the myotome, the adult zebrafish has at least 400 slow muscle fibers in each myotome. We are beginning to characterize this growth. We have shown that new slow muscle fibers are added beginning at about 24h of development, very shortly after embryonic slow muscle fibers form. Surprisingly, these added fibers develop independently of Hh signaling, and independently of the presence of embryonic slow muscle fibers (Barresi, et al., 2001).
We have proposed that the dermomyotome is an ancient and conserved structure that evolved prior to the last common ancestor of all vertebrates (Devoto, et al., 2006). We suspect that it has played a very important role in the evolution of vertebrate morphology. We recently demonstrated the existence of the dermomyotome by showing that cells on the external surface of the embryonic myotome differentiate into muscle fibers during the earliest period of myotome growth (Stellabotte, et al., 2007). These cells express the transcription factor Pax7. The zebrafish dermomyotome, like that of amniotes, is directly regulated by Hh signaling (Feng, et al, 2006). See Stellabotte and Devoto, 2007 for a review of the teleost dermomyotome.
Other aspects of somite patterning
In addition to the myotome, the somite gives rise to the
vertebrae, the ribs and (in chick) the scapula. These tissues all
develop from the sclerotome, which is a relatively small portion
of the somite in zebrafish. We are examining the factors that
regulate sclerotome development in zebrafish. Our working
hypothesis is that initial sclerotome development is not dependent
on midline signaling, but that sclerotome maintenance is.
- muscular dystrophy strikes 1 in 4,000 boys in the U.S.
- heart disease accounts for over one-third of all deaths in
the U.S.
- 150,000 babies are born with birth defects each year in the
U.S.
- cancer accounts for one-quarter of all deaths in the
U.S.
Our research into muscle fiber type development in zebrafish has been funded very generously by The Donaghue Foundation, The March of Dimes, and the National
Institutes of Health. Our current funding is through NIH grants 5R01HD044929-04 (Selecting for novel Hedgehog signaling mutations), and 2R01HD037509-06A2 (Development of muscle fiber type identity).
At its most fundamental, our research is aimed at understanding
why and how cells do what they do. How do they "decide" whether to
divide or become specialized? How do cells decide how often to
divide? How do they decide which of several possible
specializations to acquire? Answers to these questions are
necessary for designing appropriate and effective treatments for
all of the above diseases.
More specifically, if we can understand how muscle development
is triggered, and how specific types of muscle fibers are formed,
then we can start to intelligently design therapies that will not
only trigger muscle regeneration, but also trigger the appropriate
specializations in regenerated tissues that are critical for
recovery from heart disease and muscular dystrophy.
 This cross section of a 24 hour old zebrafish embryo was stained with three antibodies to identify muscle fiber types. A monolayer of embryonic slow muscle fibers (yellowish green) is on the surface of the myotome, surrounding the deeper fast muscle fibers (red). All muscle nuclei are teal. Other nuclei in the embryo are labeled blue.
This cross section of a 24 hour old zebrafish embryo was stained with three antibodies to identify muscle fiber types. A monolayer of embryonic slow muscle fibers (yellowish green) is on the surface of the myotome, surrounding the deeper fast muscle fibers (red). All muscle nuclei are teal. Other nuclei in the embryo are labeled blue.